Writer: Jess Mackin // Illustrator: Brendan Capey // Editor: Erin Pallott
The most common icebreaker in research is to ask “What do you work on?”. When I tell a researcher my topic, NF-κB signalling, I am met with the joking question “Is that a sorority?”. I cannot blame them, it does sound like it! I simply shrug and say trust me; the actual name does not help (Nuclear Factor kappa-light-chain-enhancer of activated B cells), plus it is just a mouthful!
NF-κB is not a singular thing but rather a family of five structurally related transcription factors RelA (p65), RelB, c-Rel, p100/p52 and p105/p50. In mammals, they are ubiquitously expressed as inactive dimers and upon activation they are responsible for driving physiological processes to maintain homeostatic signalling. Although they are grouped together, NF-κB can be activated and classified into two different signalling pathways: canonical and non-canonical.
Canonical NF-κB (p65, c-Rel and p105/p50) is rapidly and transiently activated by an array of stimuli, typically driving the expression of inflammatory, survival, and developmental signalling molecules. Whereas non-canonical NF-κB (RelB and p100/p52) is slowly and persistently activated by limited stimuli and typically drives the expression of immune regulators. I will be covering the former in this blog.
Typical activation of canonical NF-kB
In your cells, the most common form of NF-κB (p65-p50 dimer) is constitutively expressed in the cytoplasm in an inactive state. A protein called IκB (inhibitor of κB) protein, hides the nuclear localisation sequence of NF-kB to prevent nuclear entry and subsequent gene expression. NF-κB-IκB complexes remain in the cytoplasm of the cell waiting for an event to happen, such as a microbe entering your body, ready to initiate signalling events to maintain cellular homeostasis.
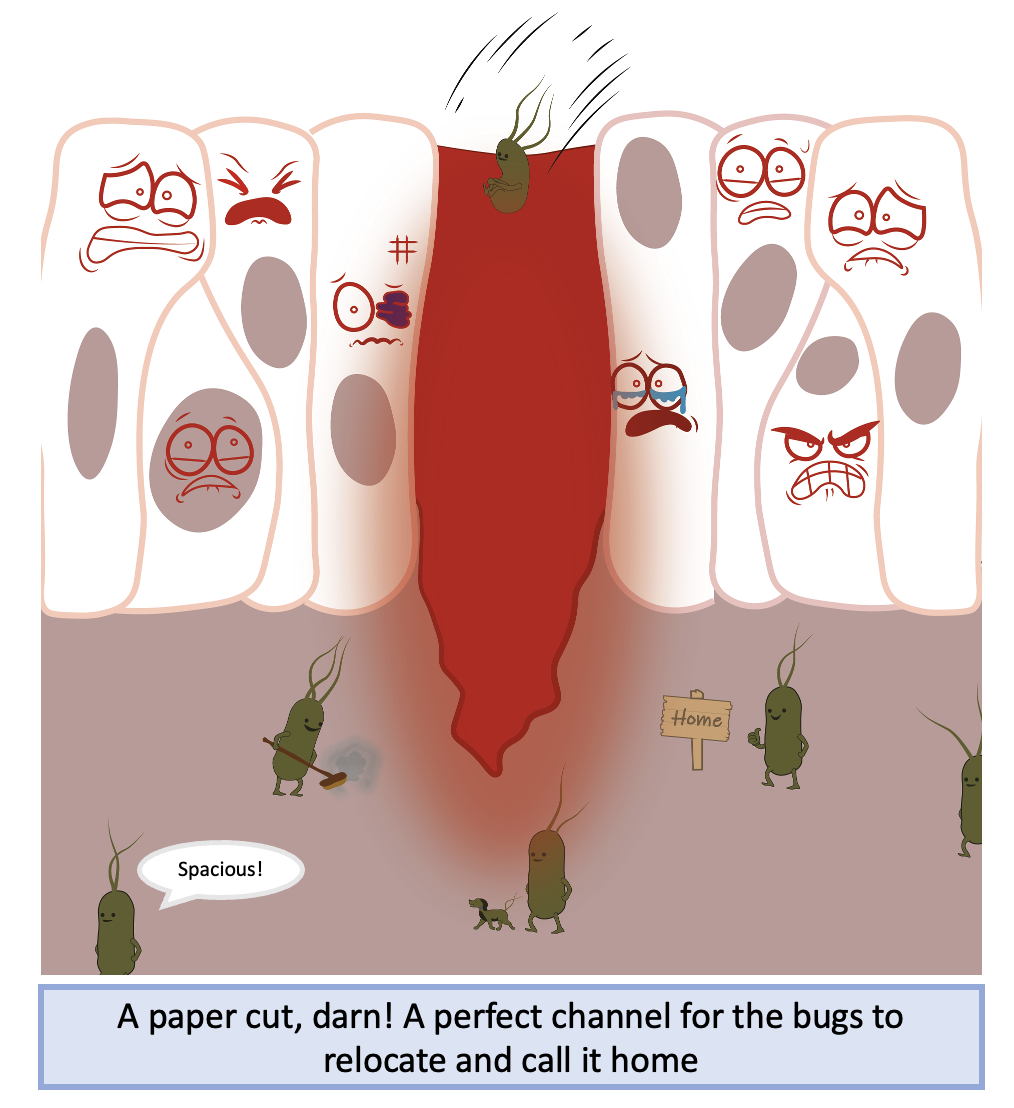
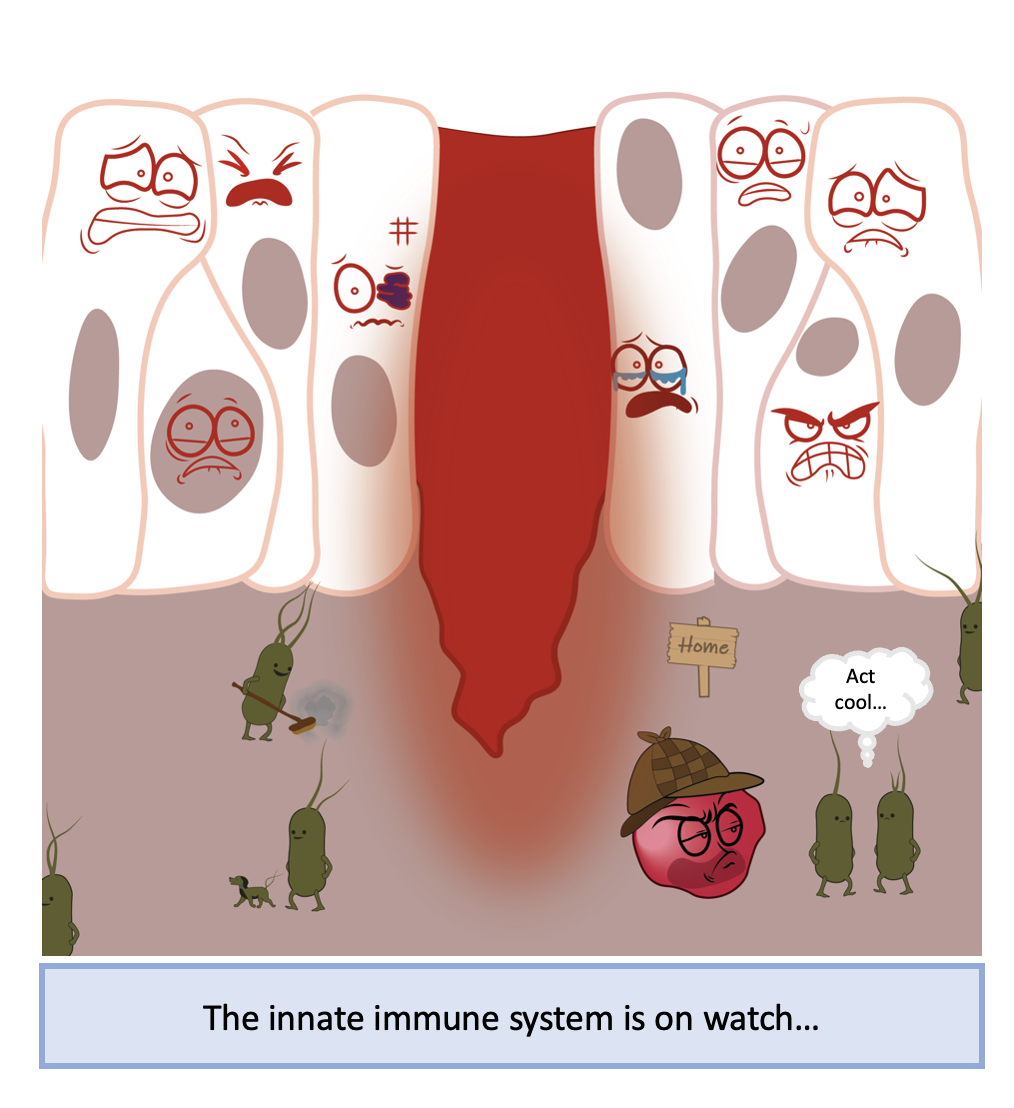
Imagine you have a paper cut which has damaged your skin, leading to an open channel for microbes to enter and make it their home. Your innate immune system passes by and interacts with these pathogens and recognises they should not be there. Pathogens have specific molecular patterns which make them recognisably foreign to your body. These molecules activate a series of receptors called pattern recognition receptors (PRRs) that lead to the activation of NF-κB. Once activated, PRRs recruit upstream intracellular signalling proteins that activate a complex of kinases called the IKK complex. The IKK complex phosphorylates IκB proteins, targeting them for degradation and freeing NF-κB to enter the nucleus.
Once in the nucleus, NF-κB binds to target recognition sites on DNA and upregulates target genes including cytokines such as interleukins and tumour necrosis factor (TNF), and chemokines such as CXCL proteins. These cytokines and chemokines enable recruitment of more immune cells which act to eradicate the microbe from your body, allowing for repair of the damage your skin has faced. NF-κB also has a role in the development and maturation of the adaptive immune system, the immune system branch that acts to protect the body from any potential future infections. In an ideal world, the innate immune response and subsequent inflammation would be transient and shut down quickly. Otherwise, it could lead to out-of-control signalling, damage to healthy tissues, and potentially disease.
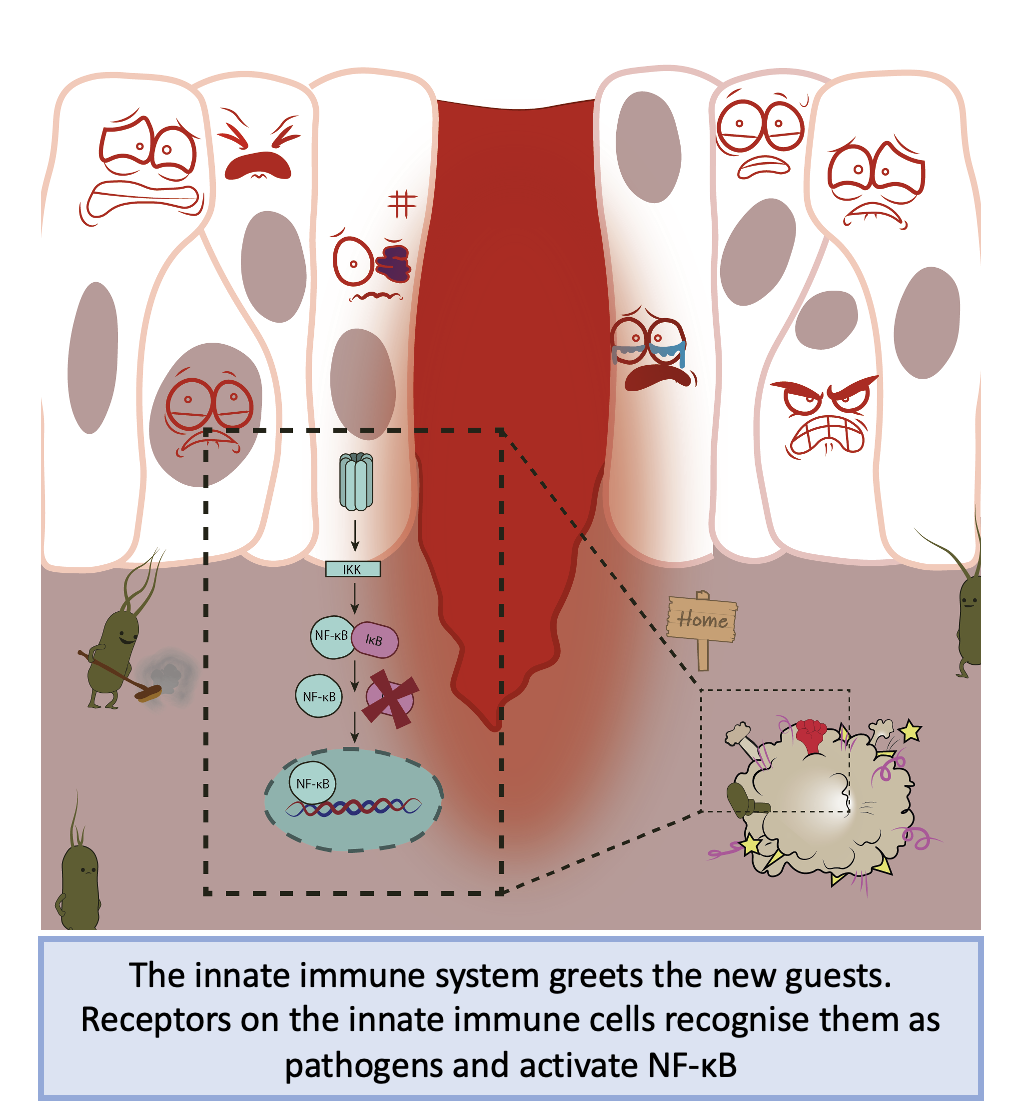
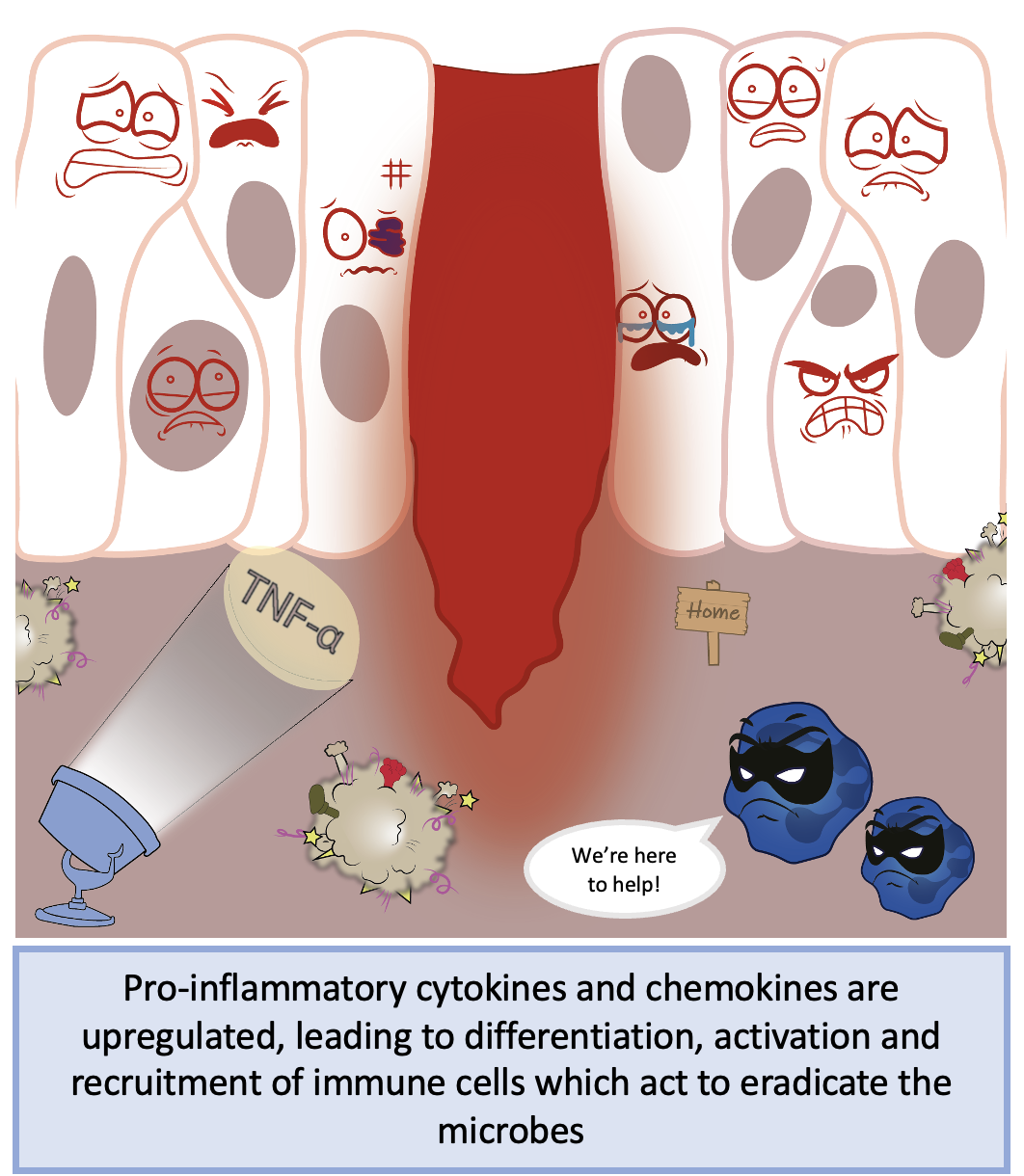
Simplistic overview of NF-κB activation during acute injury and inflammation. Figure created by Brendan Capey
Once in the nucleus, active NF-κB has the potential to transcribe over 500 different genes! Therefore, regulation of its activation is key to maintaining homeostasis and preventing excess signalling. To ensure a correct response and to prevent an imbalance in signalling, the NF-κB system establishes several autoregulatory feedback loops which act to either prevent or propagate the NF-κB response. These feedback loops ensure the response is transient and has its desired effect.
When NF-κB upregulates the expression of cytokines which elicit responses from immune cells, they bind to their respective receptors and ultimately lead to further activation of NF-κB, generating a positive feedback loop. Cytokines are able to act in an autocrine (on the cell that produced the cytokine) or in a paracrine manner (in neighbouring cells that did not make the cytokine) and are important for regulating innate and adaptive immune responses. However, the overproduction of these NF-κB positive regulators can become very dangerous in situations such as sepsis and cytokine storm (aggressive response to inflammation). Therefore, there must be a way to shut down NF-κB signalling to prevent this!
The most well known negative regulator of NF-κB are the previously mentioned IκB proteins, these are under NF-κB control and establish a negative autoregulatory feedback loop. IκB protein is newly synthesised in NF-κB active cells and binds to NF-κB in the nucleus, transporting it back into the cytoplasm in an inactive state. This, in effect, replaces the constitute IκB protein that was degraded when NF-κB was initially activated. Another negative regulator is the anti-inflammatory protein A20. This acts on upstream signalling events to prevent activation of the IKK complex and subsequent degradation of IκB. This in effect, prolongs the association of the IκB-NF-κB complexes.
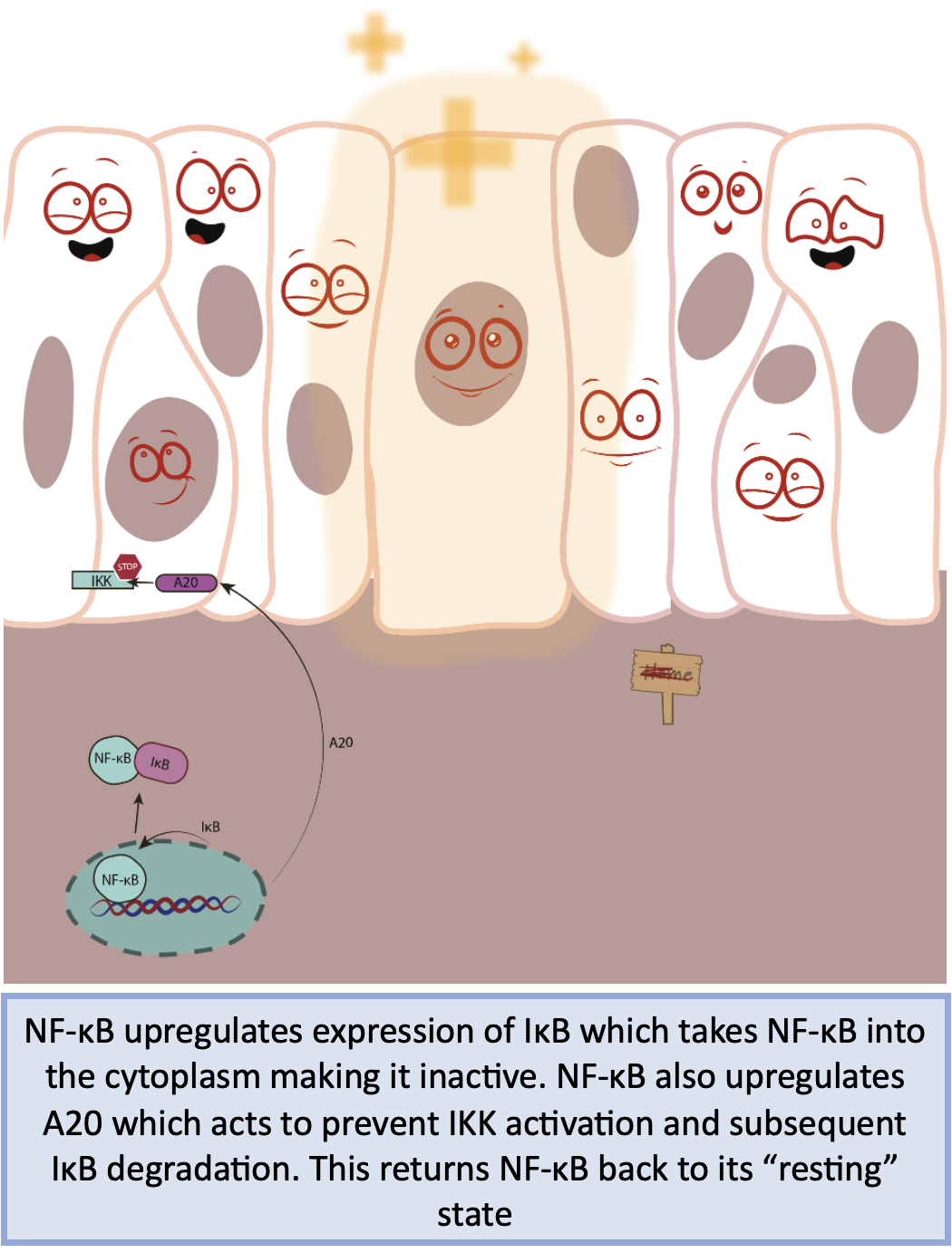
Simplistic overview of autoregulatory negative feedback of the NF-kB pathway. Figure created by Brendan Capey
When NF-kB signalling goes wrong and how we can manage it
As mentioned, NF-κB has a key role in regulating the acute inflammatory response. Inflammation is a protective mechanism employed by the body to prevent any potential damage by injury or infection. Protective inflammation is rapid and transient to prevent accumulation of cytokines and chemokines which would otherwise cause harm. Prolonged activation of the inflammatory response can lead to destruction of healthy tissues and can also cause disease if left untreated.
There are over 50 diseases that stem from dysregulated canonical NF-kB signalling, which are typically caused by alterations in NF-κB, IκB, IKK or A20 signalling. These include, but are not limited to, rheumatoid arthritis, asthma, allergies, multiple sclerosis, and cancer. As the NF-κB signalling axis is known to be aberrantly activated, there are targeted treatment strategies for patients with rheumatoid arthritis (anti-TNF drug Enteracept) and cancer (protease inhibitors such as Bortezomib).
Aside from the specialised medicine for the treatment of chronic disease, there are drugs that target NF-κB signalling and are readily available. This includes the non-steroidal anti-inflammatory aspirin and the steroid dexamethasone which can be used to moderately reduce inflammatory symptoms and relieve pain.
Due its central role in regulating over 500 genes, NF-κB cannot be targeted directly as you risk the emergence of other symptoms and a new disease state. Therefore, drugs targeting modulators of the system are favourable.
Although NF-κB has been studied for over 35 years, there is still a lot we do not understand. Namely, the in depth role of A20 in inflammation, how perturbations in environment modulate the NF-κB response and how cells mediate cross-talk of pro-inflammatory signals. A greater understanding of these and similar areas could provide insight into inflammatory diseases and help with developing better treatment strategies.
Discover more from Research Hive
Subscribe to get the latest posts to your email.
